SCIENTIFIC INFORMATION
EGCG as an Antioxidant
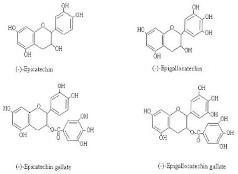
Green tea has four catechin antioxidants (see chemical structures below). The most active antioxidant is (-)-epigallocatechin gallate (EGCG), that has the most phenolic –OH groups, therefore, is used as the surrogate standard for setting its medicinal quality.
The phenolic groups of EGCG serve as electron donors in the sense that they can give up an electron or a hydrogen atom containing an electron without robbing an electron from another atom. These phenolic molecules are capable of making internal adjustment to stabilize their unpaired electron after losing a hydrogen atom. Physiological concentrations of EGCG can prevent free radical-induced chromosomal damage. The two-step hydrogen donation by a phenolic antioxidant is illustrated as follows.
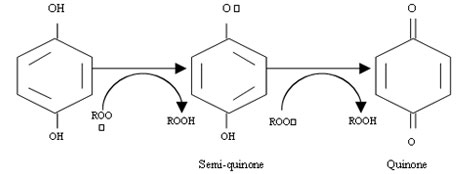
ROO. is a lipid free radical with an unpaired electron. After accepting hydrogen atom, it becomes a stable ROOH with a stable electron pair. The phenolic radical (-O.) is relativerly unreactive before forming a quinone.
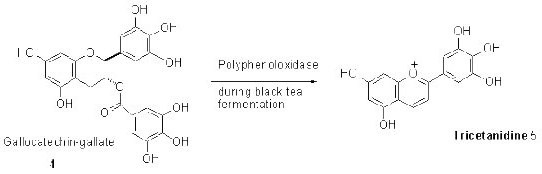
Oxidation of EGCG can occur in the dry tea leaves and in the tea liquid. The first step of oxidation is degradation of its EGCG while the green tea turns darker in color due to formation of thearubigens shown in the following reaction.
(Formation of Thearubigens)
Synonyms: N-Ethyl-L-glutamine; L-Glutamic acid gamma-(ethylamide)
Molecular structure:
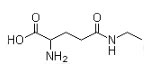
L-theanine is a special tea amino acid and a biochemical modulator. It has been shown to boost general health.